Lewy body

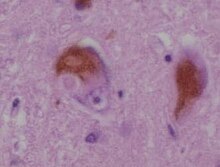
Lewy bodies are the inclusion bodies – abnormal aggregations of protein – that develop inside neurons affected by Parkinson's disease (PD), the Lewy body dementias (Parkinson's disease dementia and dementia with Lewy bodies (DLB)), and some other disorders.[which?] They are also seen in cases of multiple system atrophy, particularly the parkinsonian variant (MSA-P).[1]
Lewy bodies appear as spherical masses in the cytoplasm that displace other cell components. For instance, some Lewy bodies tend to displace the nucleus to one side of the cell.[2][better source needed] There are two main kinds of Lewy bodies – classical and cortical. A classical Lewy body is an eosinophilic cytoplasmic inclusion consisting of a dense core surrounded by a halo of 10 nm wide radiating fibrils, the primary structural component of which is alpha-synuclein (α-synuclein). While similar in many other respects, cortical Lewy bodies are only faintly eosinophilic, do not have a surrounding halo, and do not show a radial filamentous substructure.[3] Lewy bodies may be found in the midbrain (within the substantia nigra) or within the cortex.
History
[edit]In 1910, Fritz Heinrich Lewy was studying in Berlin for his doctorate.[4] He was the first doctor to notice some unusual proteins in the brain, comparing them to earlier findings by Gonzalo Rodríguez Lafora.[5] In 1913, Lafora described another case, and acknowledged Lewy as the discoverer, naming them cuerpos intracelulares de Lewy (intracellular Lewy bodies).[5] Konstantin Nikolaevich Trétiakoff found them in 1919 in the substantia nigra of PD brains, called them corps de Lewy and is credited with the eponym.[5] In 1923, Lewy published his findings in a book, The Study on Muscle Tone and Movement. Including Systematic Investigations on the Clinic, Physiology, Pathology, and Pathogenesis of Paralysis agitans.[6] Eliasz Engelhardt, who is in the neurology department at Federal University of Rio de Janeiro, argued in 2017 that Lafora should be credited with the eponym, because he named them six years before Trétiakoff.[5] Nonetheless, Trétiakoff is still the primary figure acknowledged for coining the term, "Lewy bodies".[5]
According to the Journal of the History of the Neurosciences, Dr. Lewy became interested in studying more about the brain (neurology), because of the discovery that Alois Alzheimer made in 1906. The article mentions that the third reported case of Alzheimer's disease had histological structures that happened to be similar to Lewy body histology slides, but the contribution was not given to Lewy's finding.[7]
Cell biology
[edit]
A Lewy body is composed of the protein α-synuclein associated with other proteins, such as ubiquitin,[9] neurofilament protein, and alpha B crystallin. Tau proteins may also be present, and Lewy bodies may occasionally be surrounded by neurofibrillary tangles.[10][11] Lewy bodies and neurofibrillary tangles can occasionally exist in the same neuron, particularly in the amygdala.[12]
Alpha-synuclein modulates DNA repair processes, including repair of DNA double-strand breaks (DSBs) by the process of non-homologous end joining[13] The repair function of alpha-synuclein appears to be greatly reduced in Lewy body bearing neurons, and this reduction may trigger cell death. Mutations are the reason behind their damaged repair function.[14] One mutation in particular, in the gene encoding for presynaptic alpha-synuclein, was found to have been passed down from family members with PD.[14] Similarly in regards to DLB, Lewy bodies retrieved from DLB brains were found to contain alpha-synuclein proteins that were shortened by mutations.[14]
Lewy bodies are believed to represent an aggresome response in the cell.[15] When misfolded proteins aggregate, or clump together, many diseases are more likely to develop, including those that are associated with Lewy bodies.[16] Aggregation is believed to occur when there is a high amount of misfolded proteins in the ubiquitin-proteasome pathway, which are then brought to a resulting aggresome so they can be organized into one place.[16] Since Lewy bodies are made of ubiquitinated proteins that would be handled in the ubiquitin-proteasome pathway, they may be made from this or a similar process if the pathway capacity is indeed exceeded by misfolded proteins that aggregate together.[16] Accordingly, the aggresome, where the damaged proteins fully aggregate, is akin to the Lewy body.
Despite their differences, there is evidence that a particular protein family, called 14-3-3, plays a role in the formation of both cortical and classical Lewy bodies.[17][better source needed] This makes it an important protein family in regards to Lewy body-associated diseases, and there are at least 7 forms of it that have been clearly identified in mammals.[17]
Cortical Lewy bodies are also composed of alpha-synuclein fibrils, but are less defined and lack halos. This kind of Lewy body is one of those aforementioned that regularly displaces the nucleus.[2] In histopathology, cortical Lewy bodies are a distinguishing feature for dementia with Lewy bodies, but may occasionally be seen in ballooned neurons characteristic of behavioural variant frontotemporal dementia and corticobasal degeneration,[18] as well as in patients with other tauopathies.[19]
Lewy neurites
[edit]
Lewy neurites are abnormal neurites in diseased neurons, containing granular material and abnormal α-synuclein filaments similar to those found in Lewy bodies.[20] Like Lewy bodies, Lewy neurites are a feature of α-synucleinopathies such as dementia with Lewy bodies, Parkinson's disease, and multiple system atrophy.[21] They are also found in the CA2-3 region of the hippocampus in Alzheimer's disease.[21]
See also
[edit]References
[edit]- ^ Jellinger KA (September 2007). "More frequent Lewy bodies but less frequent Alzheimer-type lesions in multiple system atrophy as compared to age-matched control brains". Acta Neuropathologica. 114 (3): 299–303. doi:10.1007/s00401-007-0227-4. PMID 17476513. S2CID 32406286.
- ^ a b Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M (August 1997). "Alpha-synuclein in Lewy bodies". Nature. 388 (6645): 839–840. Bibcode:1997Natur.388..839G. doi:10.1038/42166. PMID 9278044. S2CID 4419837.
- ^ Mrak, Robert E.; Griffin, W Sue T (October 2007). "Dementia with Lewy bodies: Definition, diagnosis, and pathogenic relationship to Alzheimer's disease". PubMed Central. Retrieved October 23, 2024.
- ^ "Friedrich H. Lewy". Whonamedit?.
- ^ a b c d e Engelhardt E (October 2017). "Lafora and Trétiakoff: the naming of the inclusion bodies discovered by Lewy". Arquivos de Neuro-Psiquiatria (Historical article). 75 (10): 751–753. doi:10.1590/0004-282X20170116. PMID 29166468.
- ^ Engelhardt E, Gomes MD (2017). "Lewy and his inclusion bodies: Discovery and rejection". Dementia & Neuropsychologia. 11 (2): 198–201. doi:10.1590/1980-57642016dn11-020012. PMC 5710688. PMID 29213511.
- ^ García-Albea E, Pérez Trullen JM (December 2003). "The Spanish school of neurology and the first American cases of Alzheimer's disease". Journal of the History of the Neurosciences. 12 (4): 437–445. doi:10.1076/jhin.12.4.437.27919. PMID 15069873. S2CID 40698888.
- ^ Braak H, Del Tredici K, Rüb U, de Vos RA, Jansen Steur EN, Braak E (March–April 2003). "Staging of brain pathology related to sporadic Parkinson's disease". Neurobiology of Aging. 24 (2): 197–211. doi:10.1016/S0197-4580(02)00065-9. PMID 12498954. S2CID 22798538.
- ^ Engelender S (April 2008). "Ubiquitination of alpha-synuclein and autophagy in Parkinson's disease". Autophagy. 4 (3): 372–374. doi:10.4161/auto.5604. PMID 18216494.
- ^ Ishizawa T, Mattila P, Davies P, Wang D, Dickson DW (April 2003). "Colocalization of tau and alpha-synuclein epitopes in Lewy bodies". Journal of Neuropathology and Experimental Neurology. 62 (4): 389–397. doi:10.1093/jnen/62.4.389. PMID 12722831.
- ^ Arima K, Hirai S, Sunohara N, Aoto K, Izumiyama Y, Uéda K, Ikeda K, Kawai M (October 1999). "Cellular co-localization of phosphorylated tau- and NACP/alpha-synuclein-epitopes in lewy bodies in sporadic Parkinson's disease and in dementia with Lewy bodies". Brain Research. 843 (1–2): 53–61. doi:10.1016/S0006-8993(99)01848-X. PMID 10528110. S2CID 11144367.
- ^ Schmidt ML, Martin JA, Lee VM, Trojanowski JQ (1996). "Convergence of Lewy bodies and neurofibrillary tangles in amygdala neurons of Alzheimer's disease and Lewy body disorders". Acta Neuropathologica. 91 (5): 475–481. doi:10.1007/s004010050454. PMID 8740227. S2CID 19770377.
- ^ Schaser AJ, Osterberg VR, Dent SE, Stackhouse TL, Wakeham CM, Boutros SW, et al. (July 2019). "Alpha-synuclein is a DNA binding protein that modulates DNA repair with implications for Lewy body disorders". Scientific Reports. 9 (1): 10919. Bibcode:2019NatSR...910919S. doi:10.1038/s41598-019-47227-z. PMC 6662836. PMID 31358782.
- ^ a b c Baba M, Nakajo S, Tu PH, et al. (April 1998). "Aggregation of alpha-synuclein in Lewy bodies of sporadic Parkinson's disease and dementia with Lewy bodies". The American Journal of Pathology. 152 (4): 879–884. PMC 1858234. PMID 9546347.
- ^ Tanaka M, Kim YM, Lee G, Junn E, Iwatsubo T, Mouradian MM (February 2004). "Aggresomes formed by alpha-synuclein and synphilin-1 are cytoprotective". The Journal of Biological Chemistry. 279 (6): 4625–4631. doi:10.1074/jbc.M310994200. PMID 14627698.
- ^ a b c Johnston JA, Ward CL, Kopito RR (December 1998). "Aggresomes: a cellular response to misfolded proteins". The Journal of Cell Biology. 143 (7): 1883–1898. doi:10.1083/jcb.143.7.1883. PMC 2175217. PMID 9864362.
- ^ a b Kawamoto Y, Akiguchi I, Nakamura S, Honjyo Y, Shibasaki H, Budka H (March 2002). "14-3-3 proteins in Lewy bodies in Parkinson disease and diffuse Lewy body disease brains". Journal of Neuropathology and Experimental Neurology. 61 (3): 245–253. doi:10.1093/jnen/61.3.245. PMID 11895039.
- ^ Dickson DW, Feany MB, Yen SH, Mattiace LA, Davies P (1996). "New Trends in the Diagnosis and Therapy of Non-Alzheimer's Dementia". Journal of Neural Transmission. Supplementum: Cytoskeletal pathology in non-Alzheimer degenerative dementia: New lesions in Diffuse Lewy body disease, Pick's disease, and Corticobasal Degeneration. Journal of Neural Transmission Supplement. 47: 31–46. doi:10.1007/978-3-7091-6892-9_2. ISBN 978-3211828236. PMID 8841955.
- ^ Popescu A, Lippa CF, Lee VM, Trojanowski JQ (December 2004). "Lewy bodies in the amygdala: increase of alpha-synuclein aggregates in neurodegenerative diseases with tau-based inclusions". Archives of Neurology. 61 (12): 1915–1919. doi:10.1001/archneur.61.12.1915. PMID 15596612.
- ^ Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M (May 1998). "alpha-Synuclein in filamentous inclusions of Lewy bodies from Parkinson's disease and dementia with lewy bodies". Proceedings of the National Academy of Sciences of the United States of America. 95 (11): 6469–6473. Bibcode:1998PNAS...95.6469G. doi:10.1073/pnas.95.11.6469. PMC 27806. PMID 9600990.
- ^ a b Marui W, Iseki E, Kato M, Akatsu H, Kosaka K (August 2004). "Pathological entity of dementia with Lewy bodies and its differentiation from Alzheimer's disease". Acta Neuropathologica. 108 (2): 121–128. doi:10.1007/s00401-004-0869-4. PMID 15235805. S2CID 22624886.
